Exploring the correlation between temperature and vitamin C content in orange juice
- medicusweblog
- Nov 1, 2024
- 14 min read
Ruby Haryanto
Introduction
My mom would always buy oranges for their vitamin C content, keeping them in our refrigerator. The reason for this was quite simple, it allowed the fruits to be fresh for longer periods of time, preserving for the vitamins and other nutrients within it as well. Whenever my grandmother would visit, she would always scold us for keeping our fruit in the fridge, insisting they should be kept outside at room temperature instead. These opposing ideas are what led to my interest in exploring which temperature the vitamin C content in oranges would be most optimal. By using a variety of temperatures, I came up with the research question: How does temperature variation (0°C, 10°C, 20°C, 30°C, 40°C) impact the vitamin C content in Almarai orange juice, measured through a redox titration using iodine solution?
Background Information
Ascorbic acid (C6H8O6) or Vitamin C, is a 6-carbon compound, bonded through hydrogen bonds, London dispersion forces, and dipole-dipole bonds (National Center for Biotechnology Information). The vitamin is essential for growth, the maintenance of bones, teeth, and more. This essential vitamin cannot be internally synthesized by humans, therefore being something we have to ingest. Vitamin C can be found in foods such as oranges, lemons, kale, and are also sold as dietary supplements. However, Vitamin C can easily be destroyed from exposure to air, light, or heat (Harvard School of Public Health).

The concentration of ascorbic acid within the orange juice can be calculated by performing a redox titration reaction using an iodine solution and a starch indicator. A redox reaction, short for reduction-oxidation reaction, are reactions in which reactants undergo changes in their oxidation states. An oxidation reaction, refers to a substance's loss of electrons, and a reduction reaction refers to substances gaining electrons (Byju). Substances that readily accept electrons in redox reactions are known as oxidizing agents, while those that readily donate electrons are referred to as reducing agents, as they tend to undergo oxidation. In this context, ascorbic acid is the reducing agent, and iodine is the oxidizing agent. The chemical equation of this reaction can be seen below:

When iodine is titrated, ascorbic acid oxidizes, forming dehydroascorbic acid, and the molecules of iodine are reduced to iodine ions. Iodine is a relatively insoluble molecule, but when iodine (I2) is complexed with iodide (I-), it forms a more soluble triiodide molecule, I2. As long as there is any ascorbic acid present, the iodine will continue to reduce iodide. Gradually, once all ascorbic acid molecules are oxidized, excess iodine molecules are then available to react with the cornstarch indicator, leading to a blue-black color complex to form. Iodine molecules appear violet in the absence of starch. When iodine molecules interact with starch, their electrons undergo transitions from lower to higher energy levels, causing them to absorb orange light in the visible spectrum. Since orange light is absorbed by the iodine-starch complex, the wavelength absorbed is reflected as blue to the human eye. The blue-black color complex is the signal for the end point of the reaction (Goedecke).
Vitamin C is a highly sensitive compound vulnerable to denaturation, influenced by various factors such as heat, light, and air. It is hypothesized that raising the temperature will lead to a decrease in vitamin C content in the orange juice samples, as shown in figure 2. As temperature rises, it escalates the kinetic energy of molecules, consequently increasing the frequency of collisions between the ascorbic acid molecules and the triiodide molecules. Given that the titration reaction consumes ascorbic acid, an
elevated reaction rate results in more ascorbic acid being depleted within a specific timeframe. This implies that storing orange juice at higher temperatures could expedite the degradation of its vitamin C content.
Variables
Independent variable: Temperature of orange juice (5°C, 12°C, 20°C, 30°C, 40°C)
Dependent variable: Volume of iodine solution titrated into the solution
Controlled variables:
1. Freshness of orange juice:
To maintain consistency in the freshness of the orange juice, it is essential to control factors that can affect its concentration, such as packaging and storage conditions. The orange juice used in the experiment was purchased the day before from the same store, ensuring identical production and expiry dates. A 300 cm3 bottle of orange juice was chosen to minimize variations in freshness. To prevent oxidation, sample preparation was performed immediately before titrations. After extracting the required sample, the orange juice bottle was promptly sealed and returned to the refrigerator.
2. The Orange juice brand:
Using a consistent orange juice brand is crucial because different brands may have varying vitamin C concentrations due to processing and sourcing. These concentration variations directly influence the frequency of molecular collisions during titration, leading to inaccuracies in my data. For example, a brand with a higher vitamin C concentration would lead to more rapid titration reactions compared to a brand with lower vitamin C content. I decided to buy the almarai brand orange juice with no pulp for this
experiment. This enables me to accurately assess the sole influence of temperature, without introducing other brand-related disparities.
3. The concentration of iodine solution used for titration:
The concentration of the iodine solution used for titration should be kept constant throughout the experiment, ensuring that the same amount of titrant is applied across all trials. Deviating from a consistent concentration could lead to variations in the amount of iodine available for the reaction with ascorbic acid, thereby affecting the reliability and comparability of the results. For instance, if the iodine concentration were too low, it could lead to incomplete reactions and a falsely high estimation of vitamin C content. Conversely, if the concentration were too high, it could result in excessive iodine usage, leading to an underestimate of the actual vitamin C content. I will create a big batch of a 0.01M iodine solution to ensure all trials consume the same concentration level.
Materials & Apparatus:
- 300 cm3 of orange juice (no pulp)
- 0.8 grams of potassium iodide
- 0.6 grams of iodine
- 2 grams of cornstarch
- 750 cm3 of distilled water
- 250 cm3 conical flask ± 5.00
- 600 cm3 beaker ± 5.00
- 100 cm3 graduated cylinder ± 5.00
- Digital scale ± 1.00
- Hot plate
- Weighing boat
- Burette ± 0.05
- Pipette
Safety: Vitamin C solutions, while not typically harmful, may cause mild skin or eye irritation upon contact, so it is advisable to wear safety goggles throughout the experiment. In case of contact with the eyes, rinse thoroughly for at least 10 minutes. If contacted with the skin, thoroughly wash affected areas with soap and water to alleviate irritation. The use of iodine solution, being an irritant, can pose moderate risks as it is potentially harmful if ingested, inhaled, or in contact with the skin or eyes. It can also possibly stain skin or clothes if come into contact with. If it comes in contact with the skin or eyes, rinse the area with soap and water, and rinse eyes for at least 10 minutes. Extra caution is necessary when handling iodine solution, and a well-ventilated workspace or a fume hood should be used to minimize inhalation risks. To avoid environmental hazards and further safety hazards, ensure proper disposal of chemicals into designated waste beakers after each trial.
Procedure:
Preparation of the iodine solution (0.01 mol dm-3):
1. Use a digital scale and a weight boat to measure 0.8 grams of potassium iodide, and place it into a dry, 500 cm3 beaker
2. Use the same materials to measure 0.6 grams of iodine, and place in the same beaker as the potassium iodide
3. Measure 150 cm3 of distilled water with the use of a graduated cylinder, and add into the beaker containing the iodine and iodide
4. Gently stir the solution with a glass stirring rod until dissolved
5. Once dissolved, add 350 cm3 of distilled water, measured with the use of a 500 cm3 graduated cylinder
Preparation of starch indicator solution (0.01 mol dm-3):
6. Use a digital scale and a weight boat to measure 2 grams of cornstarch
7. In a new 250 cm3 beaker, add the measured cornstarch
8. Measure 100 cm3 of close to boiling water, and pour into the breaker
9. Stir continuously with the use of a glass stirring rod
10. Once the starch completely dissolves, leave solution to cool
Preparation of the burette:
11. Locate a retort stand and place it onto the laboratory table
12. Attach a clean burette onto a burette clamp by placing it between the clamp’s jaws
13. Slide the clamp onto the stands vertical rod, and secure firmly
14. Ensure that the burette is closed, stopcock perpendicular to the burette
15. Measure 10 cm3 of the 0.01 mol dm-3 Iodine solution using a graduated cylinder, and pour into the burette
16. Rinse the inside of the burette with the 10 cm3 0.01 mol dm-3 iodine solution, and pour out into a 100 cm3 waste beaker
17. Pour the 0.01 mol dm-3 iodine solution into the burette to slightly above the 0.0 cm3mark at the top
18. Open the stopcock slowly to release the air bubbles at the bottom of the burette, and close the stopcock again
Titration of the room temperature (20°C) orange juice sample and preparation of 5°C and 12°C samples:
19. Measure 200 cm3 of orange juice using a graduated cylinder and pour into a beaker
20. Place the beaker with 200 cm3 of orange juice into the fridge, and leave to chill
21. Leave the rest of the bottle of orange juice in classroom to rest at room temperature
22. Measure 20 cm3 of orange juice using a graduated cylinder, and pour into a 250 cm3 conical flask
23. With the use of a transfer pipette, add 2 drops of the cornstarch solution into the conical flask containing the orange juice, and stir using a glass rod
24. Place the conical flask containing the orange juice-starch solution under the burette
25. Record the initial volume of iodine solution in the burette
26. Slowly titrate the iodine solution into the orange juice-starch solution, while swirling the flask
27. Close the stopcock when the color of the solution within the conical flask turns blue-black color, indicating the endpoint of the titration.
28. Record the final volume of the iodine solution
29. Pour the chemicals within the flask into a waste beaker
30. Rinse the flask thoroughly with water before proceeding to the next trial.
31. Repeat steps 20-28 until 5 trials are completed and recorded
Titration of the 30°C, 40°C vitamin C sample:
32. Measure 100 cm3 of room temperature of orange juice using a graduated cylinder, and pour into a beaker
33. Turn on the hot plate, and place the beaker containing 100 cm3 of orange juice onto the hot plate
34. Place a thermometer into the beaker, and watch the temperature rise until 30°C is reached
35. Once 30°C is reached, take the beaker off of the hot plate and measure 20 cm3 of the heated orange juice using a 100 cm3 graduated cylinder
36. Pour the heated 20 cm3 of orange juice into a 250 cm3 conical flask
37. Repeat steps 21-28 until 5 trials are completed
a. Keep watch on the temperature of the remainder of the heated orange juice in the beaker
b. If temperature drops, place the beaker back onto the hot plate to reheat the orange juice to designated temperature
38. Repeat steps 30-35 but for temperature of 40°C
Titration of the 5°C and 12°C vitamin C samples:
39. Take the 200 cm3 of chilled orange juice out of the fridge
40. Measure 100 cm3 of orange juice using a graduated cylinder and pour into a separate 100 beaker
41. Place the remaining orange juice into the freezer
42. Place a thermometer into the 100 cm3 of orange juice and wait until the temperature reaches 12°C
43. Once the temperature reaches 12°C, measure 20 cm3 of orange juice with the use of a 50 cm3 graduated cylinder and pour into a 250 cm3 conical flask
44. Repeat steps 21-28 until 5 trials are completed
c. Keep watch on the temperature of the remainder of the chilled orange juice in the beaker
d. If temperature rises above designated temperature, place the beaker back into the fridge to cool it down again
45. Take the remaining 100 cm3 of orange juice 5°C out of the freezer
46. Measure 20 cm3 of orange juice with the use of a 50 cm3 graduated cylinder, and pour into a 250 cm3 conical flask
47. Repeat step 42
Data:
Raw Data



Qualitative observations:
● Flask became foggy through condensation
● Dark blue from the starch and iodine reaction makes the orange color turn green
● As more iodine was added into the solution, the orange color became more dull and greener, with a tint of
black
● Grayish-Green becomes the endpoint

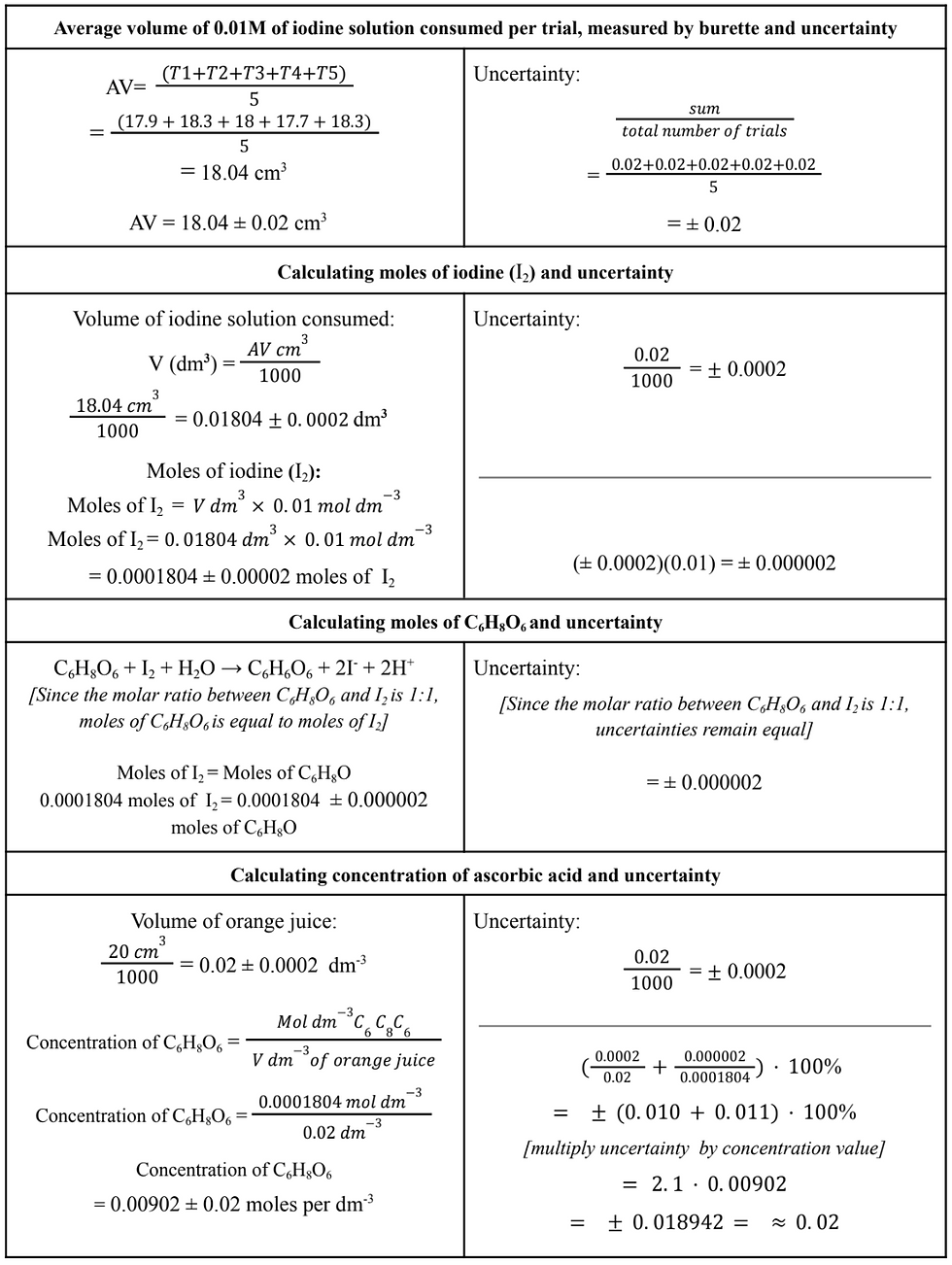

Conclusion
Exploring the effects of temperature on the ending concentration of ascorbic acid in the orange juice solution, the collected data conclusively demonstrates a consistent decrease in ascorbic acid concentration with rising temperatures. As the temperature of the orange juice increased, the concentration of the ascorbic acid content decreased,consistently presenting inverse correlation between these variables.. In the redox reaction, iodine acts as an oxidizing agent, converting ascorbic acid to its oxidized form,
dehydroascorbic acid, with concurrent reduction of iodine to iodide ions. The temperature-dependent behavior of this reaction, as explained by the Arrhenius equation, shows that higher temperatures increase kinetic energy, leading to more frequent and successful collisions and faster ascorbic acid oxidation. This can be seen in my data as the higher the temperature of the orange juice resulted in a lower concentration of ascorbic acid. As seen in table 3, the highest temperature, 40.00°C, had the lowest concentration of ascorbic acid at 5.22 x 10-3mol dm-3, 42% less than the lowest temperature, 5.00°C, 9.02 x 10-3mol dm-3. At 20.00°C, the middle temperature had a concentration of 7.4 x 10-3 mol, falling right in between the highest and lowest temperatures. The accelerated motion and collisions due to the increased temperatures between these molecules facilitate a more rapid conversion of ascorbic acid to its oxidized form, dehydroascorbic acid, mediated by iodine. This intensified molecular interaction due to heightened temperature results in a more efficient oxidation process, ultimately contributing to the observed
reduction in vitamin C concentration. Therefore, the results affirm that higher temperatures lead to a decrease in ascorbic acid concentration.
Evaluation
First and foremost, the use of a burette is a strength of my experiment due to its precision due to its fine graduations marked along its length, allowing measurements to the nearest 0.01 cm3. As the measured volumes of iodine consumed to reach the endpoint are used to calculate the concentration of vitamin c, any deviation from the accurate measurements could lead to significantly incorrect calculations. This precision also allows for the controlled titrant addition into the orange juice-starch solution in the flask. By cautiously adding titrant in smaller increments, particularly as the expected endpoint approaches, this method ensures an accurate determination of the reaction’s endpoint (blue-black complex) while minimizing overshooting. The finely calibrated scale of the burette also allows for the accurate measurement of the volume of titrant added during the titration process. If the measurement of volume isn't precise, the accuracy of calculating the concentration becomes compromised, as the measurement errors would propagate through the concentration calculations, leading to unreliable outcomes.
Another strength in my experiment, specifically as a titration, is its inherent replicability. Titration experiments, characterized by precise and standardized procedures, ensure ease of replication. The straightforwardness of titration procedures imposes no constraints on replication, making it feasible to duplicate the experiment with minimal limitations, thus further ensuring the reliability and consistency of the obtained data. This replicability of the experiment also allows for the consistent recreation of the
experiment's steps, enabling accurate comparisons.
Throughout the experiment, there were errors that could have impacted the data that I collected. For example, one error that resulted from the experiment was the fluctuation and inconsistencies in temperature. For the independent variable, I chose to change the temperature of the orange juice to show the effects of different temperatures on the ending ascorbic acid content.
Each temperature would have 5 trials to increase the precision of my data and reduce the likelihood of an error. However, throughout the experiment, there were times where the temperatures of the orange juice would have changed. For example, this could be seen in the condensation that sat on the outside of the flask for the colder temperatures of orange juice, as well as the steam from the hotter temperatures. The flask contained more thermal energy than the 5.00°C and 12.00°C orange juice solutions. Once the colder orange juice was poured into the flask, the flask immediately fogged up, covered in condensation, showing a heat transfer between the room temperature flask, and the cold orange juice. Heat flows naturally from the warmer environment to the cooler one, transferring heat from the flask to the orange juice. Eventually reaching the thermal equilibrium, the temperature of the
orange juice would increase, creating temperature variations between the trials. Similar occurrences were observed for the temperatures 30.00°C and 40.00°C. Condensation on the outside of the flask, as well as steam, caused a heat transfer between the heated orange juice and the room temperature flask, decreasing the temperature of the overall trial temperatures of the system. However, this heat transfer is inevitable as steam will always escape into the environment, but the heat transfer between the orange juice and the flask can be decreased by heating or cooling the flask to be the same temperature as the orange juice measured, decreasing the change in temperature, ensuring more precise data. Furthermore, the speed at which the titration was executed influenced the extent of heat exchange between the orange juice and the flask. During this time frame, significant heat loss or gain could have occurred, particularly in the final moments, titrating at a slower pace.
Another systematic error that could have impacted my data is the measurement of the starch, or rather the lack thereof. The role of the starch solution is to serve as an indicator, making it possible to detect the endpoint of the titration. The choice of putting in 2 drops of starch solution rather than measuring out a specific volume of starch to add into the orange juice solution could lead to inconsistencies in the volume of starch solution added. Variations in the amount of starch indicator added could lead to outliers in my data, affecting the reliability of my results. If there were an increased amount of starch indicator added, it could potentially influence the reliability of the results by affecting the measured endpoint in the titration process. For instance, an excess of starch indicator might lead to an overstated volume of titrant needed to reach the endpoint. This would occur because the surplus indicator could react with the iodine titrant beyond the point of complete reaction with the orange juice solution.
The inconsistencies in starch indicator quantities lead to certain titrations consuming either excessive or insufficient iodine. Consequently, this results in variable volumes of iodine measured at the endpoint as the observed endpoint would appear earlier than the actual equivalence point, leading to an overestimation of the ascorbic acid concentration. The imprecise endpoint detection, exacerbated by inconsistent drop sizes, causes data points to deviate from the expected trend. Instead of using conventional pipettes, micropipettes offer increased precision in measurements, allowing for the controlled and uniform delivery of small liquid volumes. This would make the addition of starch into the system more consistent, therefore making my experimental data more reliable. However, despite the errors that impacted the accuracy and precision of my data, the magnitude
that it impacted my data was minimal. As seen in figure 3, my r2 value was significantly high, at 0.991. This value, approaching the maximum of 1, indicates an exceptional fit of the data to the established trendline in the graph. Approximately 99.1% of the variability in the ascorbic acid concentration can be attributed to changes in temperature and such a strong correlation reaffirms the accuracy and consistency of my data. The minimal error bars also seen in figure 3 indicate the minimal variation due to error in my experiments measurements. Together, the high r2 value and consistently narrow error bars validate the reliability of my data, as well as robustness of the conclusion that temperature and ascorbic acid concentration have an inverse relationship.
An extension to the experiment involves exploring the impact of pH variations on the stability of ascorbic acid in orange juice, furthering the understanding of its degradation. The experiment might entail adjusting the pH of orange juice samples to different levels, such as acidic, neutral, and alkaline conditions. This investigation into pH-dependent degradation rates would offer valuable insights into optimal pH conditions for preserving vitamin C content in orange juice and aid in determining suitable
storage or processing methods to retain its nutritional value.
Works Cited
Byju, Akash. “Redox Reactions - Examples, Types, Applications, Balancing.” BYJU'S, 2022, https://byjus.com/jee/redox-reactions/. Accessed 7 November 2023.
Goedecke, Catharina. “Why Does Iodine Turn Starch Blue?” ChemistryViews, 6 December 2016, https://www.chemistryviews.org/details/education/10128441/Why_Does_Iodine_Turn_Starch_Blue/. Accessed 7 November 2023.
Harvard School of Public Health. “Vitamin C | The Nutrition Source | Harvard T.H. Chan School of Public Health.” Harvard T.H. Chan School of Public Health, March 2023, https://www.hsph.harvard.edu/nutritionsource/vitamin-c/. Accessed 4 September 2023.
National Center for Biotechnology Information. “Ascorbic Acid | HC6H7O6 | CID 54670067.” PubChem, 2022, https://pubchem.ncbi.nlm.nih.gov/compound/Ascorbic-Acid. ''''''''''''''''''Accessed 5 September 2023.
Comments